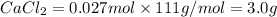Chemistry, 03.07.2020 04:01 marissarsmith8205
Determine the quantity (g) of pure CaCl2 in 7.5 g of CaCl2•9H2O. Show your work.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3
You know the right answer?
Determine the quantity (g) of pure CaCl2 in 7.5 g of CaCl2•9H2O. Show your work....
Questions

Mathematics, 01.11.2020 14:30


Social Studies, 01.11.2020 14:40

English, 01.11.2020 14:40

Social Studies, 01.11.2020 14:40

History, 01.11.2020 14:40


Advanced Placement (AP), 01.11.2020 14:40

Mathematics, 01.11.2020 14:50

Social Studies, 01.11.2020 14:50

Mathematics, 01.11.2020 14:50




Mathematics, 01.11.2020 14:50

Mathematics, 01.11.2020 14:50


History, 01.11.2020 14:50


Social Studies, 01.11.2020 14:50

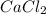 in 7.5 g of
in 7.5 g of 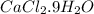 is 3.0 g
is 3.0 g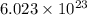 of particles.
of particles.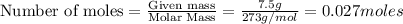
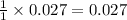 mole of
mole of