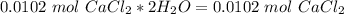Chemistry, 02.07.2020 03:01 Fangflora3
calculate how many moles of CaCl2•2H2O are present in 1.50 g of CaCl2•2H2O and then calculate how many moles of pure CaCl2 are present in the 1.50 g of CaCl2•2H2O.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
calculate how many moles of CaCl2•2H2O are present in 1.50 g of CaCl2•2H2O and then calculate how ma...
Questions



Mathematics, 02.06.2021 06:00


Mathematics, 02.06.2021 06:00

Mathematics, 02.06.2021 06:00



Social Studies, 02.06.2021 06:00

English, 02.06.2021 06:00

History, 02.06.2021 06:00

Mathematics, 02.06.2021 06:00

Mathematics, 02.06.2021 06:00



Biology, 02.06.2021 06:00

Mathematics, 02.06.2021 06:00


Mathematics, 02.06.2021 06:00

Social Studies, 02.06.2021 06:00

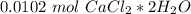
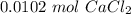
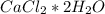 . For this, we have to know the atomic mass of each atom:
. For this, we have to know the atomic mass of each atom: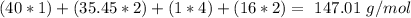
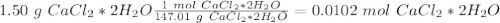
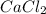 . Therefore, we have a 1:1 mol ratio . With this in mind, we will have the same number of moles for
. Therefore, we have a 1:1 mol ratio . With this in mind, we will have the same number of moles for