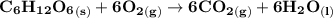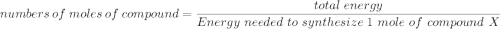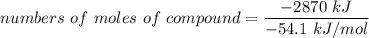Chemistry, 02.07.2020 22:01 treypickich14
Assume that the complete combustion of one mole of glucose, a monosaccharide, to carbon dioxide and water liberates 2870 kJ2870 kJ of energy (ΔG°′=−2870 kJ/mol(ΔG°′=−2870 kJ/mol ). If the energy generated by the combustion of glucose is entirely converted to the synthesis of a hypothetical compound X, calculate the number of moles of the compound that could theoretically be generated. Use the value ΔG°′compound X=−54.1 kJ/molΔG°′compound X=−54.1 kJ/mol . Round your answer to two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Assume that the complete combustion of one mole of glucose, a monosaccharide, to carbon dioxide and...
Questions

Mathematics, 19.01.2021 21:40

English, 19.01.2021 21:40


Health, 19.01.2021 21:40





Mathematics, 19.01.2021 21:40

Biology, 19.01.2021 21:40






French, 19.01.2021 21:40

History, 19.01.2021 21:40


Social Studies, 19.01.2021 21:40

Mathematics, 19.01.2021 21:40

 53 mole
53 mole