
Chemistry, 02.07.2020 09:01 luvpeaceandsocc3678
Consider the chemical reaction below. Zn(s) + 2H+(aq) -> Zn2+(aq) + H2(g). Which half reaction correctly represents reduction for this equation?
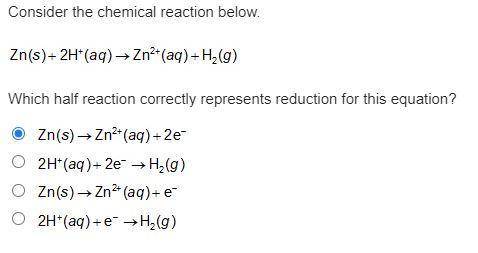

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1


Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Consider the chemical reaction below. Zn(s) + 2H+(aq) -> Zn2+(aq) + H2(g). Which half reaction co...
Questions

Social Studies, 25.11.2019 10:31

Mathematics, 25.11.2019 10:31



Physics, 25.11.2019 10:31

Mathematics, 25.11.2019 10:31

Mathematics, 25.11.2019 10:31


History, 25.11.2019 10:31

Mathematics, 25.11.2019 10:31




English, 25.11.2019 10:31


Mathematics, 25.11.2019 10:31


English, 25.11.2019 10:31


Mathematics, 25.11.2019 10:31



