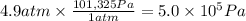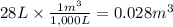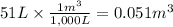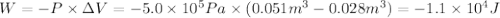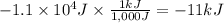Chemistry, 01.07.2020 23:01 thecoolgirl02
Calculate the work (kJ) done during a reaction in which the internal volume expands from 28 L to 51 L against an outside pressure of 4.9 atm. Calculate the work (kJ) done during a reaction in which the internal volume expands from 28 L to 51 L against an outside pressure of 4.9 atm. 11 kJ -11 kJ -39 kJ 39 kJ 0 kJ; No work is done.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Calculate the work (kJ) done during a reaction in which the internal volume expands from 28 L to 51...
Questions





Mathematics, 10.06.2020 16:57


Mathematics, 10.06.2020 16:57








Mathematics, 10.06.2020 16:57

Chemistry, 10.06.2020 16:57

Mathematics, 10.06.2020 16:57

Mathematics, 10.06.2020 16:57

Mathematics, 10.06.2020 16:57