Chemistry, 01.07.2020 15:01 bluetigerbird4745
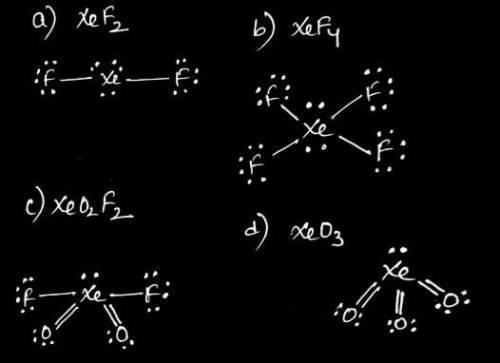
Of the noble gases, only Kr, Xe, and Rn are known to form a few compounds with O and/or F. Complete the following Lewis structures by adding all of the necessary lone pairs and any multiple bonds for each molecule below. Draw your structure with the lowest possible formal charges.
A) XeF2
B) XeF4
C) XeO2F2
D) XeO3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1
You know the right answer?
Of the noble gases, only Kr, Xe, and Rn are known to form a few compounds with O and/or F. Complete...
Questions

Geography, 29.09.2019 15:10

Mathematics, 29.09.2019 15:10

Mathematics, 29.09.2019 15:10


Mathematics, 29.09.2019 15:10


Geography, 29.09.2019 15:10

Biology, 29.09.2019 15:10

Computers and Technology, 29.09.2019 15:10

History, 29.09.2019 15:10


English, 29.09.2019 15:10


Mathematics, 29.09.2019 15:10


Computers and Technology, 29.09.2019 15:10

Mathematics, 29.09.2019 15:10

Biology, 29.09.2019 15:10