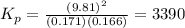Chemistry, 29.06.2020 20:01 lflugo6oyn4sp
At a particular temperature, an equilibrium mixture the reaction below was found to contain 0.171 atm of I2, 0.166 atm of Cl2 and 9.81 atm of ICl. Calculate the value of the equilibrium constant, Kp at this temperature. I2(g) + Cl2(g) <=> 2 ICl(g)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
At a particular temperature, an equilibrium mixture the reaction below was found to contain 0.171 at...
Questions


Biology, 14.01.2021 17:30




Mathematics, 14.01.2021 17:40

Mathematics, 14.01.2021 17:40

World Languages, 14.01.2021 17:40

History, 14.01.2021 17:40

Mathematics, 14.01.2021 17:40

Mathematics, 14.01.2021 17:40

World Languages, 14.01.2021 17:40



History, 14.01.2021 17:40


Mathematics, 14.01.2021 17:40

Mathematics, 14.01.2021 17:40

Mathematics, 14.01.2021 17:40

Chemistry, 14.01.2021 17:40

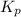 .
.![K_{p} =\frac{[ICl]^2}{[I_{2}][Cl_{2}] }](/tpl/images/0696/9637/8ab45.png)