Chemistry, 29.06.2020 20:01 mdaniella522
At a given temperature, 3.12 atm of H2 and 5.52 atm of I2 are mixed and allowed to come to equilibrium. The equilibrium pressure of HI is found to be 1.738 atm. Calculate Kp for the reaction at this temperature. H2(g) + I2(g) <=> 2 HI(g). Give your answer to 3 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
At a given temperature, 3.12 atm of H2 and 5.52 atm of I2 are mixed and allowed to come to equilibri...
Questions



English, 09.06.2021 09:50


History, 09.06.2021 09:50


English, 09.06.2021 09:50

Social Studies, 09.06.2021 09:50

Chemistry, 09.06.2021 09:50


English, 09.06.2021 09:50


Physics, 09.06.2021 09:50

History, 09.06.2021 09:50

English, 09.06.2021 09:50

Social Studies, 09.06.2021 09:50

History, 09.06.2021 14:00

Mathematics, 09.06.2021 14:00

Mathematics, 09.06.2021 14:00

Mathematics, 09.06.2021 14:00

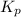 . To do so, we will need to use the ICE chart. The I in ICE is initial quantity. In this case, it is the initial pressure. Pressure is in atm. The C in ICE is change in each quantity. The E is equilibrium.
. To do so, we will need to use the ICE chart. The I in ICE is initial quantity. In this case, it is the initial pressure. Pressure is in atm. The C in ICE is change in each quantity. The E is equilibrium.![K_{p} =\frac{[HI]^2}{[H_{2}][I_{2} ] }](/tpl/images/0696/9799/d09a4.png)