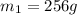Chemistry, 30.06.2020 18:01 6710000831
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 28.0∘C. What is the mass of the silver block?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated...
Questions






Biology, 05.12.2020 02:30

Mathematics, 05.12.2020 02:40






French, 05.12.2020 02:40


Spanish, 05.12.2020 02:40

Mathematics, 05.12.2020 02:40

Mathematics, 05.12.2020 02:40


Mathematics, 05.12.2020 02:40

Mathematics, 05.12.2020 02:40

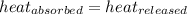
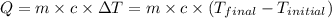
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0697/4196/09236.png) .................(1)
.................(1)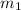 = mass of silver = ?
= mass of silver = ?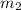 = mass of water = 100.0 g
= mass of water = 100.0 g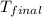 = final temperature =
= final temperature = 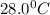
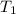 = temperature of silver =
= temperature of silver = 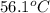
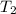 = temperature of water =
= temperature of water = 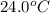
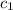 = specific heat of silver =
= specific heat of silver = 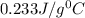
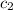 = specific heat of water=
= specific heat of water= 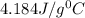
![-m_1\times 0.233\times (28.0-56.1)=[100.0\times 4.184\times (28.0-24.0)]](/tpl/images/0697/4196/c0833.png)