
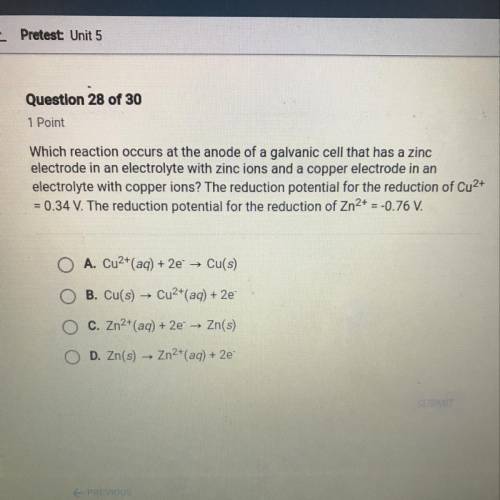
Which reaction occurs at the anode of a galvanic cell that has a zinc
electrode in an electrolyte with zinc ions and a copper electrode in an
electrolyte with copper ions? The reduction potential for the reduction of Cu2+
= 0.34 V. The reduction potential for the reduction of Zn2+ = -0.76 V.

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1

Chemistry, 23.06.2019 08:30
Of the following elements, which is the least reactive? a. c b. h c. li d. he
Answers: 1
You know the right answer?
Which reaction occurs at the anode of a galvanic cell that has a zinc
electrode in an electrolyte w...
Questions



Social Studies, 13.04.2021 04:20

History, 13.04.2021 04:20

Business, 13.04.2021 04:20

Social Studies, 13.04.2021 04:20

Computers and Technology, 13.04.2021 04:20

Mathematics, 13.04.2021 04:20




Computers and Technology, 13.04.2021 04:20


Computers and Technology, 13.04.2021 04:20








