Chemistry, 28.06.2020 23:01 maustin5323
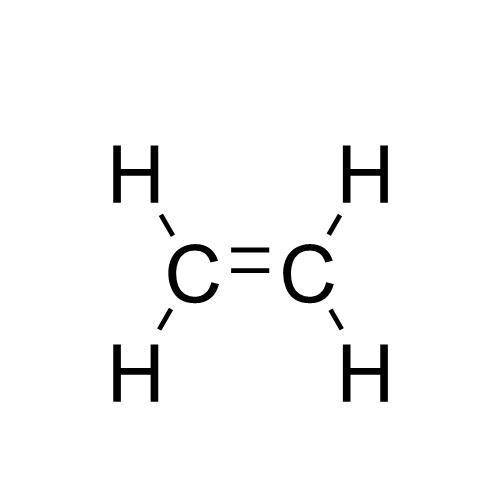
A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of the compound.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2


Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Wri...
Questions

Mathematics, 28.05.2020 21:04

History, 28.05.2020 21:04

Mathematics, 28.05.2020 21:04

Arts, 28.05.2020 21:04

English, 28.05.2020 21:04


Mathematics, 28.05.2020 21:04


Mathematics, 28.05.2020 21:04

Computers and Technology, 28.05.2020 21:04



Chemistry, 28.05.2020 21:05

Mathematics, 28.05.2020 21:05




History, 28.05.2020 21:05

Chemistry, 28.05.2020 21:05