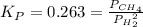C(s) + 2 H2(g) = CH,(9)
has Kp = 0.263 at 1000. K. Calculate the total pressure at equilibrium when 5.207 g of H, and 23.29 g of C(s) are placed in
a 9.32 L flask and heated to 1000, K.
atm
Protal =
Calculate the total pressure when 5.207 g of H, and 8.333 g of C(s) are placed in a 9.32 L flask and heated to 1000. K.
atm
Ptotal =

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
C(s) + 2 H2(g) = CH,(9)
has Kp = 0.263 at 1000. K. Calculate the total pressure at equilibrium when...
Questions

Mathematics, 15.12.2021 04:00




Mathematics, 15.12.2021 04:00




Social Studies, 15.12.2021 04:00



Mathematics, 15.12.2021 04:00



Social Studies, 15.12.2021 04:00

Biology, 15.12.2021 04:00



Social Studies, 15.12.2021 04:00

English, 15.12.2021 04:00