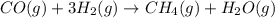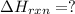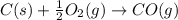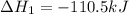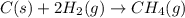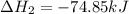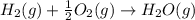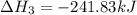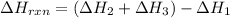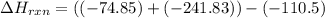Chemistry, 29.06.2020 03:01 priscillavaladez1112
Use Hess's law and the following equations to calculate the ΔHreaction for the reaction CO(g) + 3H2(g) CH4(g) + H2O(g). (Show your work.) (4 points) • C(s) + O2(g) CO(g) ΔH = –110.5 kJ • C(s) + 2H2(g) CH4(g) ΔH = –74.85 kJ • H2(g) + O2(g) H2O(g) ΔH = –241.83 kJ

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Use Hess's law and the following equations to calculate the ΔHreaction for the reaction CO(g) + 3H2(...
Questions

Mathematics, 19.02.2020 04:02








Mathematics, 19.02.2020 04:02


Mathematics, 19.02.2020 04:02

Mathematics, 19.02.2020 04:02




History, 19.02.2020 04:02




Computers and Technology, 19.02.2020 04:02