
Chemistry, 27.06.2020 18:01 my7butterflies
Calculate ΔG (in kJ) for the following reaction at 1.0 atm for C2H6, 0.5 atm for O2, and 2.0 atm for CO2, and 25 oC: C2H6 (g) + O2 (g) ---> CO2 (g) + H2O (l) (unbalanced) ΔGfo C2H6 (g) = - 32.89 kJ/mol; ΔGfo CO2 (g) = - 394.4 kJ/mol; ΔGfo H2O (l) = - 237.13 kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Calculate ΔG (in kJ) for the following reaction at 1.0 atm for C2H6, 0.5 atm for O2, and 2.0 atm for...
Questions

English, 29.04.2021 17:40

Mathematics, 29.04.2021 17:40


Mathematics, 29.04.2021 17:40


Biology, 29.04.2021 17:40





Mathematics, 29.04.2021 17:40



Mathematics, 29.04.2021 17:40



Advanced Placement (AP), 29.04.2021 17:40

Mathematics, 29.04.2021 17:40



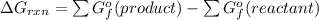
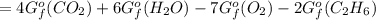
![[4(-394.4)+6(-237.13)-7(0)-2(-32.89)]kJ/mol\\\\=-1577.6-1422.78+65.78\\\\=-3000.38+65.78\\\\=-2934.6kJ/mol](/tpl/images/0695/9673/9ec14.png)



