
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and in a coffee-cup calorimeter. In one measurement, qrxn=−13.3qrxn=−13.3 kJkJ in the other qrxn=−11.9qrxn=−11.9 kJkJ. Which value was obtained in the bomb calorimeter? (Assume that the reaction has a positive ΔVΔV in the coffee-cup calorimeter.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
The same reaction, with exactly the same amount of reactant, is conducted in a bomb calorimeter and...
Questions

Mathematics, 09.09.2021 18:10

Health, 09.09.2021 18:10


World Languages, 09.09.2021 18:10

Mathematics, 09.09.2021 18:20

English, 09.09.2021 18:20

Mathematics, 09.09.2021 18:20


Mathematics, 09.09.2021 18:20

Health, 09.09.2021 18:20




English, 09.09.2021 18:20

Mathematics, 09.09.2021 18:20

English, 09.09.2021 18:20

Engineering, 09.09.2021 18:20

English, 09.09.2021 18:20

Chemistry, 09.09.2021 18:20

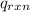 = -13.3 KJ belongs to the bomb calorimeter
= -13.3 KJ belongs to the bomb calorimeter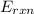 of the reaction, the work of the reaction
of the reaction, the work of the reaction 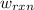 and the heat of the reaction
and the heat of the reaction 

