
Chemistry, 27.06.2020 02:01 jennymares
Consider the equation below. CaCO3(S) CaO(S) + CO2(g) What is the equilibrium constant expression for the given reaction?
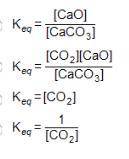
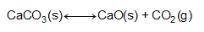

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
Consider the equation below. CaCO3(S) CaO(S) + CO2(g) What is the equilibrium constant expression fo...
Questions

Mathematics, 11.12.2019 07:31


Mathematics, 11.12.2019 07:31

Mathematics, 11.12.2019 07:31

Biology, 11.12.2019 07:31

Social Studies, 11.12.2019 07:31



Spanish, 11.12.2019 07:31

Mathematics, 11.12.2019 07:31


Mathematics, 11.12.2019 07:31

Geography, 11.12.2019 07:31


Geography, 11.12.2019 07:31







