
Chemistry, 27.06.2020 02:01 sairaanwar67
Consider the following reversible reaction. CO(g)+2H2(g) CH3OH(g) What is the equilibrium constant expression for the given system?
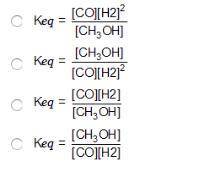
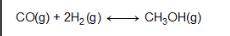

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1


Chemistry, 23.06.2019 10:00
Abike ride event is 30 miles. a first aid tent is put at the 3/4 mark of the course. how many miles from the starting point is the first aid tent?
Answers: 1

You know the right answer?
Consider the following reversible reaction. CO(g)+2H2(g) CH3OH(g) What is the equilibrium constant e...
Questions


Business, 20.10.2021 14:00


Computers and Technology, 20.10.2021 14:00




English, 20.10.2021 14:00


Biology, 20.10.2021 14:00

Chemistry, 20.10.2021 14:00


Business, 20.10.2021 14:00






History, 20.10.2021 14:00



