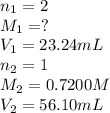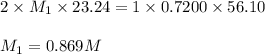Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1


Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
You know the right answer?
23.24 mL of a solution of the acid H2SO4 is titrated, and 56.10 mL of 0.7200-M NaOH is required to r...
Questions


Mathematics, 23.10.2020 17:30

Mathematics, 23.10.2020 17:30


Mathematics, 23.10.2020 17:30

Social Studies, 23.10.2020 17:30


Mathematics, 23.10.2020 17:30

Social Studies, 23.10.2020 17:30


Social Studies, 23.10.2020 17:30

Mathematics, 23.10.2020 17:30

Business, 23.10.2020 17:30

English, 23.10.2020 17:30

Computers and Technology, 23.10.2020 17:30

History, 23.10.2020 17:30




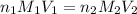
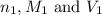 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 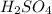
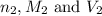 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.