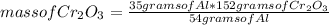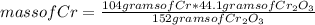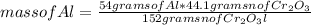Chemistry, 27.06.2020 15:01 deonnaturner68p7hz7y
How many grams of Cr can be produced by the reaction of 44.1 g of Cr2O3 with 35.0 g of Al according to the following chemical equation? How many grams of the excess react will remain once the reaction goes to completion. 2Al + Cr2O3 à Al2O3 + 2Cr

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
It has been two weeks since charles met with daniel, a dietitian, who provided charles with a menu for weight loss. charles and his mother are going back to see daniel again with a chart of the food charles has eaten. the following lists what charles ate in one day: breakfast 1 banana, 1 cup of nonfat milk, 1 egg lunch 1 cup of carrots, 3 oz of steak, 1 apple, 1 cup of nonfat milk dinner 6 oz of skinless chicken, 1 baked potato, 3 oz of broccoli, 1 cup of nonfat milk
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
How many grams of Cr can be produced by the reaction of 44.1 g of Cr2O3 with 35.0 g of Al according...
Questions


Biology, 08.03.2021 06:10

English, 08.03.2021 06:10


Mathematics, 08.03.2021 06:10


Mathematics, 08.03.2021 06:10


Social Studies, 08.03.2021 06:10



World Languages, 08.03.2021 06:10




Business, 08.03.2021 06:10




Mathematics, 08.03.2021 06:10