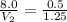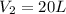Chemistry, 27.06.2020 15:01 josie17340
A 8.0 L sample at 25•C and 1.00 atm of pressure contains 0.5 moles of gas. If an additional 0.75 moles of gas at the same pressure and temperature are added, what is the final total volume of the gas?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

You know the right answer?
A 8.0 L sample at 25•C and 1.00 atm of pressure contains 0.5 moles of gas. If an additional 0.75 mol...
Questions



History, 25.08.2019 09:50

Chemistry, 25.08.2019 09:50

Social Studies, 25.08.2019 09:50

Social Studies, 25.08.2019 09:50


Mathematics, 25.08.2019 09:50

Mathematics, 25.08.2019 09:50

Health, 25.08.2019 09:50

Chemistry, 25.08.2019 09:50

English, 25.08.2019 09:50

Mathematics, 25.08.2019 09:50



Geography, 25.08.2019 09:50

Mathematics, 25.08.2019 09:50



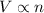
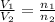
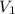 = initial volume of gas = 8.0 L
= initial volume of gas = 8.0 L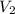 = final volume of gas = ?
= final volume of gas = ?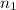 = initial moles of gas = 0.5 moles
= initial moles of gas = 0.5 moles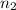 = final moles of gas = 0.5 + 0.75 = 1.25 moles
= final moles of gas = 0.5 + 0.75 = 1.25 moles