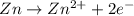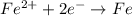Chemistry, 27.06.2020 09:01 wsdafvbhjkl
The hall reaction of an oxidation-reduction reaction shows that iron gains electrons. What does this electron gain mean for iron?
A
It is neutralized
B.
It is oxidized
C. It is reduced
D.
It has dissolved.
E
It has precipitated

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1


Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1
You know the right answer?
The hall reaction of an oxidation-reduction reaction shows that iron gains electrons. What does this...
Questions

Mathematics, 05.09.2020 02:01

Chemistry, 05.09.2020 02:01




Business, 05.09.2020 02:01




Mathematics, 05.09.2020 03:01

Mathematics, 05.09.2020 03:01


Mathematics, 05.09.2020 03:01

Social Studies, 05.09.2020 03:01

Social Studies, 05.09.2020 03:01





Mathematics, 05.09.2020 03:01