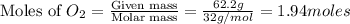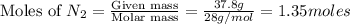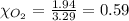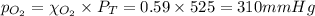Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2



Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
A gaseous mixture of O2 and N2 contains 37.8% nitrogen by mass. What is the partial pressure of oxyg...
Questions


Mathematics, 27.09.2021 14:00


Mathematics, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00


Mathematics, 27.09.2021 14:00



Chemistry, 27.09.2021 14:00

Advanced Placement (AP), 27.09.2021 14:00


Mathematics, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00




Mathematics, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00

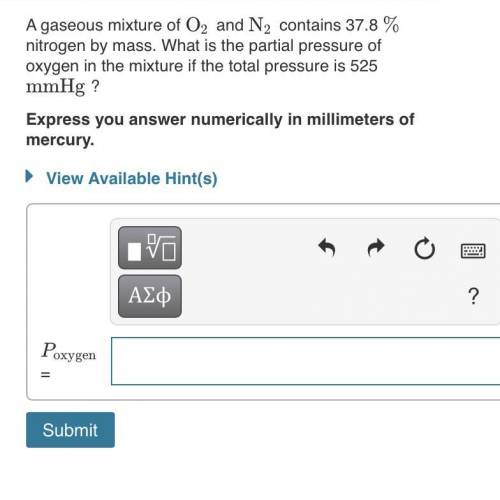
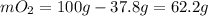
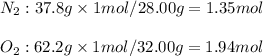
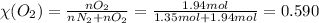
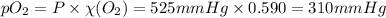
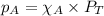
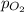 = partial pressure of
= partial pressure of 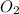 = ?
= ?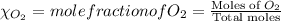
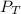 = total pressure of mixture = 525 mmHg
= total pressure of mixture = 525 mmHg