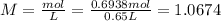Chemistry, 26.06.2020 17:01 villafana36
A 650.0 mL solution contains 125 grams of glucose (C6H12O6). If the molar mass of C6H12O6 is 180.16 g/mol, what is the molarity of this solution? answer options are 0.0106 M C6H12O6 0.0195 M C6H12O6 1.07 M C6H12O6 1.92 M C6H12O6
need help ASAP
will mark brainlest

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
A 650.0 mL solution contains 125 grams of glucose (C6H12O6). If the molar mass of C6H12O6 is 180.16...
Questions

Mathematics, 15.11.2020 02:40


Health, 15.11.2020 02:40


Mathematics, 15.11.2020 02:40

Business, 15.11.2020 02:40

Business, 15.11.2020 02:40

Mathematics, 15.11.2020 02:40



Mathematics, 15.11.2020 02:40




Business, 15.11.2020 02:40



Mathematics, 15.11.2020 02:40

Computers and Technology, 15.11.2020 02:40

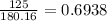 mol de glucosa
mol de glucosa