Chemistry, 26.06.2020 15:01 SLEEPSRIZK69
How many grams of chlorine gas are needed to react with 3.5 liters of a 1.7 molar
potassium bromide solution?
Cl2 + 2 KBr - 2 KCl + Br2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
How many grams of chlorine gas are needed to react with 3.5 liters of a 1.7 molar
potassium bromide...
Questions



English, 25.02.2021 20:30

English, 25.02.2021 20:30

Biology, 25.02.2021 20:30

Chemistry, 25.02.2021 20:30



History, 25.02.2021 20:30

Mathematics, 25.02.2021 20:30

Health, 25.02.2021 20:30



Mathematics, 25.02.2021 20:30






Mathematics, 25.02.2021 20:30

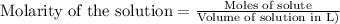 .....(1)
.....(1)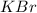 solution = 1.7 M
solution = 1.7 M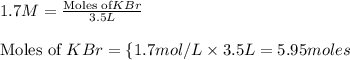
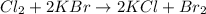
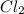
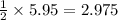 moles of
moles of