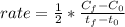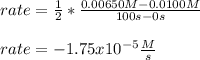Chemistry, 26.06.2020 16:01 emilyswinge4421
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2NO2 → 2NO + O2 In a particular experiment at 300 °C, [NO2] drops from 0.0100 to 0.00650 M in 100 s. The rate of disappearance of NO2 for this period is .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2NO2 → 2NO + O2 In a partic...
Questions


Physics, 08.12.2021 02:10


World Languages, 08.12.2021 02:10


Mathematics, 08.12.2021 02:10

History, 08.12.2021 02:10

SAT, 08.12.2021 02:10

Mathematics, 08.12.2021 02:10


Mathematics, 08.12.2021 02:10

History, 08.12.2021 02:10


French, 08.12.2021 02:10


Mathematics, 08.12.2021 02:10

Mathematics, 08.12.2021 02:10

Mathematics, 08.12.2021 02:10


English, 08.12.2021 02:10