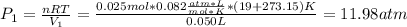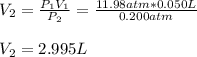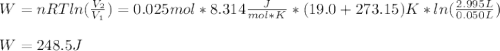A quantity of 0.0250 mol of a gas initially at 0.050 L and 19.0°C undergoes a constant-temperature expansion against a constant pressure of 0.200 atm. If the gas is allowed to expand unchecked until its pressure is equal to the external pressure, what would its final volume be before it stopped expanding, and what would be the work done by the gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
You know the right answer?
A quantity of 0.0250 mol of a gas initially at 0.050 L and 19.0°C undergoes a constant-temperature e...
Questions


Mathematics, 14.04.2021 20:50


Mathematics, 14.04.2021 20:50

Mathematics, 14.04.2021 20:50


Biology, 14.04.2021 20:50

Physics, 14.04.2021 20:50



Mathematics, 14.04.2021 20:50

Mathematics, 14.04.2021 20:50


Biology, 14.04.2021 20:50



Chemistry, 14.04.2021 20:50


Health, 14.04.2021 20:50

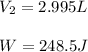
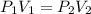
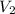 by firstly computing the initial pressure:
by firstly computing the initial pressure: