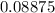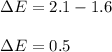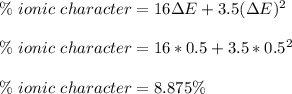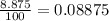Chemistry, 26.06.2020 15:01 jretes8780
The compound gallium phosphide () is a compound semiconductor having mixed ionic and covalent bonding. The electronegativities for and are 1.6 and 2.1 respectively. Calculate the fraction of the bonding that is ionic.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
The compound gallium phosphide () is a compound semiconductor having mixed ionic and covalent bondin...
Questions

Mathematics, 16.02.2021 20:20

Physics, 16.02.2021 20:20

Mathematics, 16.02.2021 20:20


Mathematics, 16.02.2021 20:20



English, 16.02.2021 20:20


History, 16.02.2021 20:20


Spanish, 16.02.2021 20:20

Mathematics, 16.02.2021 20:20

History, 16.02.2021 20:20


Biology, 16.02.2021 20:20


History, 16.02.2021 20:20


English, 16.02.2021 20:20