
Chemistry, 25.06.2020 02:01 brookcoyanpeus5y
The acetate ion is the conjugate base of the weak acid acetic acid. The value of Kb for CH3COO-, is 5.56×10-10. Write the equation for the reaction that goes with this equilibrium constant.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
The acetate ion is the conjugate base of the weak acid acetic acid. The value of Kb for CH3COO-, is...
Questions

Business, 30.09.2019 12:00

Biology, 30.09.2019 12:00

Chemistry, 30.09.2019 12:00

History, 30.09.2019 12:00

History, 30.09.2019 12:00

Mathematics, 30.09.2019 12:00

Physics, 30.09.2019 12:00


Mathematics, 30.09.2019 12:00



Biology, 30.09.2019 12:00

Physics, 30.09.2019 12:00

Social Studies, 30.09.2019 12:00



History, 30.09.2019 12:00

Social Studies, 30.09.2019 12:00


![5.56\times 10^{-10}=\frac{[CH_3COOH]}{[CH_3COO^-]\times [H^+]}](/tpl/images/0693/5990/d4718.png)
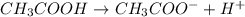
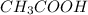 donates a proton and thus behaves as an acid and forms
donates a proton and thus behaves as an acid and forms 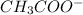 which is called as the conjugate base of
which is called as the conjugate base of 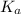 and the dissociation constant of bases is given by the term
and the dissociation constant of bases is given by the term 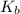 and is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios.
and is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios.![K_a=\frac{[CH_3COO^-]\times [H^+]}{[CH_3COOH]}](/tpl/images/0693/5990/0c492.png)
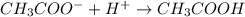
![K_b=\frac{[CH_3COOH]}{[CH_3COO^-]\times [H^+]}](/tpl/images/0693/5990/71be5.png)


