
At a certain temperature this reaction follows first-order kinetics with a rate constant of 0.0197s. 2N2O5 (g) right arrow 2N2O4 (g) + O2 (g)Suppose a vessel contains N2O5 at a concentration of 0.280 M. Calculate how long it takes for the concentration of N2Os to decrease by 0.0476 M. You may assume no other reaction is important.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
At a certain temperature this reaction follows first-order kinetics with a rate constant of 0.0197s....
Questions

Mathematics, 23.01.2020 06:31



Mathematics, 23.01.2020 06:31


Social Studies, 23.01.2020 06:31

Mathematics, 23.01.2020 06:31

Mathematics, 23.01.2020 06:31

Social Studies, 23.01.2020 06:31

Mathematics, 23.01.2020 06:31

History, 23.01.2020 06:31

Mathematics, 23.01.2020 06:31


Mathematics, 23.01.2020 06:31

History, 23.01.2020 06:31




English, 23.01.2020 06:31

Mathematics, 23.01.2020 06:31

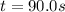
![ln(\frac{[N_2O_5]}{[N_2O_5]_0} )=-kt](/tpl/images/0693/5032/59694.png)
![t=\frac{ln(\frac{[N_2O_5]}{[N_2O_5]_0} )}{-k}=\frac{ln(\frac{0.0476M}{0.280M})}{-0.0197s}\\ \\t=90.0s](/tpl/images/0693/5032/59a2f.png)


