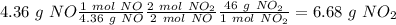Nitrogen monoxide is produced by combustion in an automobile engine. For the following reaction, 4.36 grams of nitrogen monoxide are mixed with excess oxygen gas . The reaction yields 5.46 grams of nitrogen dioxide . nitrogen monoxide ( g ) oxygen ( g ) nitrogen dioxide ( g ) What is the theoretical yield of nitrogen dioxide

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

You know the right answer?
Nitrogen monoxide is produced by combustion in an automobile engine. For the following reaction, 4.3...
Questions

English, 19.01.2021 22:10

Arts, 19.01.2021 22:10

Engineering, 19.01.2021 22:10



Mathematics, 19.01.2021 22:10

Mathematics, 19.01.2021 22:10

Mathematics, 19.01.2021 22:10




Social Studies, 19.01.2021 22:10



Mathematics, 19.01.2021 22:10

Computers and Technology, 19.01.2021 22:10

Biology, 19.01.2021 22:10

Mathematics, 19.01.2021 22:10


Biology, 19.01.2021 22:10

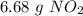
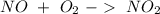
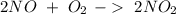
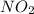 . To do this, we have to first convert the 4.36 g of
. To do this, we have to first convert the 4.36 g of 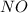 to moles
to moles