Chemistry, 23.06.2020 17:01 jluckie080117
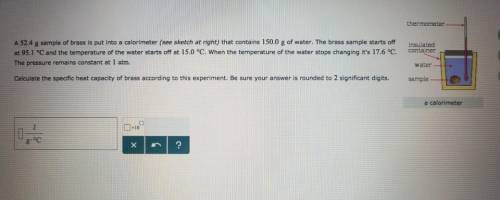
A sample of brass is put into a calorimeter (see sketch at right) that contains of water. The brass sample starts off at and the temperature of the water starts off at . When the temperature of the water stops changing it's . The pressure remains constant at . Calculate the specific heat capacity of brass according to this experiment. Be sure your answer is rounded to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2


Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
A sample of brass is put into a calorimeter (see sketch at right) that contains of water. The brass...
Questions


Mathematics, 29.06.2019 21:30

Health, 29.06.2019 21:30


Mathematics, 29.06.2019 21:30

Mathematics, 29.06.2019 21:30

Mathematics, 29.06.2019 21:30

Social Studies, 29.06.2019 21:30

Mathematics, 29.06.2019 21:30


Mathematics, 29.06.2019 21:30

History, 29.06.2019 21:30



Mathematics, 29.06.2019 21:30

Biology, 29.06.2019 21:30

English, 29.06.2019 21:30

History, 29.06.2019 21:30


Mathematics, 29.06.2019 21:30

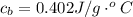
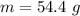
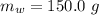
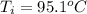
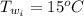
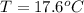
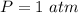
![H_L = m * c_b * [T_i - T]](/tpl/images/0692/3585/a3617.png)
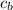 is the specific neat of the brass sample
is the specific neat of the brass sample ![H_g = m_w *c_w * [T_w - T ]](/tpl/images/0692/3585/35bc1.png)
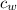 is the specific heat of water which has a constant value of
is the specific heat of water which has a constant value of 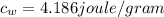
![H_L = H_g \ \equiv m* c_b * [T_i -T] = m_w * c_w * [T - T_w]](/tpl/images/0692/3585/4fcd7.png)
![52.4 * c_b * [95.1 - 17.6] = 150 * 4.186 * [ 17.6 - 15.0]](/tpl/images/0692/3585/31561.png)