Question 1:
Consider the nucleophilic substitution reaction between 1-chloro-
3,3-dimethylpen...

Chemistry, 23.06.2020 10:57 madmatt873
Question 1:
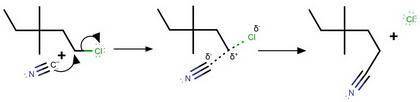
Consider the nucleophilic substitution reaction between 1-chloro-
3,3-dimethylpentane and the cyanide ion:
xa
+
ci
NC-
1-chloro-3,3-dimethylpentane
(a) Is the alkyl halide 1°, 2° or 3°?
(b) Will the nucleophilic substitution reaction be Sn1 or Sn2?
(c) Give the substitution mechanism showing all full and partial
positive and negative charges, electron movements using curved
arrows and any intermediates or transition states.
2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Questions

Mathematics, 25.01.2021 17:50


Mathematics, 25.01.2021 17:50



Mathematics, 25.01.2021 17:50


English, 25.01.2021 17:50


Computers and Technology, 25.01.2021 17:50

Mathematics, 25.01.2021 17:50

Mathematics, 25.01.2021 17:50

Mathematics, 25.01.2021 17:50


Geography, 25.01.2021 17:50

Mathematics, 25.01.2021 17:50


Mathematics, 25.01.2021 17:50

Social Studies, 25.01.2021 17:50