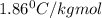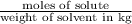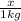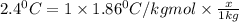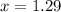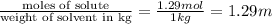Chemistry, 22.06.2020 00:57 Tianylee2328
How many moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing point of -2.4°C? The freezing point depression constant for water is 1.86°C•kg/mol. What is the molality of the solution?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
How many moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing...
Questions

Mathematics, 12.02.2021 07:40

Chemistry, 12.02.2021 07:40


History, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40



Mathematics, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40


English, 12.02.2021 07:40

English, 12.02.2021 07:40


Mathematics, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40

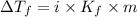
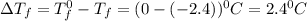 = Depression in freezing point
= Depression in freezing point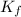 = freezing point constant for water=
= freezing point constant for water=