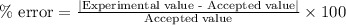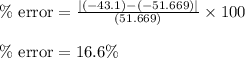Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
If the theoretical value for AH of the reaction HCl + NH 3 NH 4 Cl is -51.669 kJ/mol, but from your...
Questions



Mathematics, 01.03.2021 14:00

Social Studies, 01.03.2021 14:00






Mathematics, 01.03.2021 14:00

Mathematics, 01.03.2021 14:00

History, 01.03.2021 14:00

English, 01.03.2021 14:00

Mathematics, 01.03.2021 14:00


History, 01.03.2021 14:00


Mathematics, 01.03.2021 14:00


Mathematics, 01.03.2021 14:00