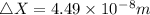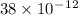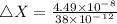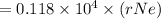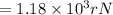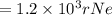An atom of helium has a radius = and an average speed in the gas phase at of . . Suppose the speed of a helium atom at has been measured to within . Calculate the smallest possible length of box inside of which the atom could be known to be located with certainty. Write your answer as a multiple of and round it to significant figures. For example, if the smallest box the atom could be in turns out to be times the radius of an atom of helium, you would enter "" as your answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

You know the right answer?
An atom of helium has a radius = and an average speed in the gas phase at of . . Suppose the speed o...
Questions

Health, 23.06.2019 13:30

Computers and Technology, 23.06.2019 13:30

Physics, 23.06.2019 13:30










Social Studies, 23.06.2019 13:30

Business, 23.06.2019 13:30





Computers and Technology, 23.06.2019 13:30

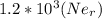
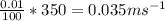 m/s
m/s
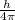
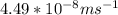
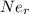 (radius of Neon) i.e.
(radius of Neon) i.e. 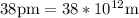
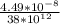
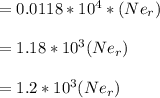
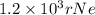
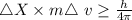 ....... (i)
....... (i)