Chemistry, 21.06.2020 01:57 nevecronin
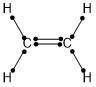
Draw a Lewis structure and indicate the hybridization for each molecule that contains more than one interior atom. Draw the Lewis structure for C2H2 (whose skeletal structure is HCCH). Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
Draw a Lewis structure and indicate the hybridization for each molecule that contains more than one...
Questions


Chemistry, 28.01.2020 13:56


Mathematics, 28.01.2020 13:56

Social Studies, 28.01.2020 13:56



Biology, 28.01.2020 13:56





English, 28.01.2020 13:56


Mathematics, 28.01.2020 13:56



History, 28.01.2020 13:56

Mathematics, 28.01.2020 13:56