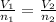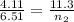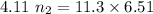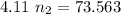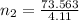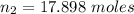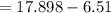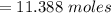Chemistry, 20.06.2020 20:57 tddreviews
A flexible container at an initial volume of 4.11 L contains 6.51 mol of gas. More gas is then added to the container until it reaches a final volume of 11.3 L. Assuming the pressure and temperature of the gas remain constant, calculate the number of moles of gas added to the container.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
A flexible container at an initial volume of 4.11 L contains 6.51 mol of gas. More gas is then added...
Questions

Mathematics, 06.03.2020 01:02

Mathematics, 06.03.2020 01:02

Social Studies, 06.03.2020 01:02