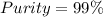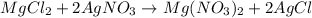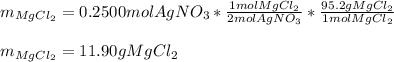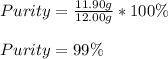Chemistry, 20.06.2020 16:57 Makoshark6887
A 12.00g sample of MgCl2 was dissolved in water. 0.2500mol of AgNO3 was required to precipitate all the chloride ions from the solution. Calculate the purity (as a mass percentage) of MgCl2 in the sample. Your answer should have four significant figures (round to the nearest hundredth of a percent).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
A 12.00g sample of MgCl2 was dissolved in water. 0.2500mol of AgNO3 was required to precipitate all...
Questions

Mathematics, 06.10.2019 13:30

Chemistry, 06.10.2019 13:30

Mathematics, 06.10.2019 13:30


Mathematics, 06.10.2019 13:30


Mathematics, 06.10.2019 13:30




Mathematics, 06.10.2019 13:30


Mathematics, 06.10.2019 13:30


Social Studies, 06.10.2019 13:30


History, 06.10.2019 13:30


Geography, 06.10.2019 13:30