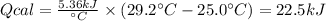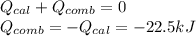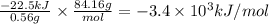Chemistry, 20.06.2020 12:57 stormserena
A 0.56 g sample of liquid C6H12 was combusted completely using excess oxygen inside a bomb (constant volume) calorimeter, with the products being carbon dioxide and liquid water. The calorimeter's heat capacity is 5.36 kJ °C-1. If the temperature inside the calorimeter increased from 25.0 °C to 29.2 °C, determine ΔrH for this reaction in kJ mol-1 (with respect to C6H12) at 298 K. Do not worry about how realistic the final answer is. You have 5 attempts at this question. TIP: To report an answer in scientific notation, enter it using the format "2.3E4", which means "2.3 x 104" (without the quotation marks)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1


Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
A 0.56 g sample of liquid C6H12 was combusted completely using excess oxygen inside a bomb (constant...
Questions





Physics, 28.08.2019 06:30

Biology, 28.08.2019 06:30

Mathematics, 28.08.2019 06:30

History, 28.08.2019 06:30


English, 28.08.2019 06:30

Mathematics, 28.08.2019 06:30








Mathematics, 28.08.2019 06:30

Mathematics, 28.08.2019 06:30

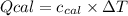
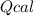 : heat absorbed by the calorimeter
: heat absorbed by the calorimeter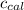 : heat capacity of the calorimeter
: heat capacity of the calorimeter : change in the temperature
: change in the temperature