Using the rules of significant figures, calculate the following: 1.2315+
0.116 + 15.10 is.
16...

Chemistry, 19.06.2020 07:57 lightbright828
Using the rules of significant figures, calculate the following: 1.2315+
0.116 + 15.10 is.
16.45
• 16.4
16
16.4475
0/3 nts
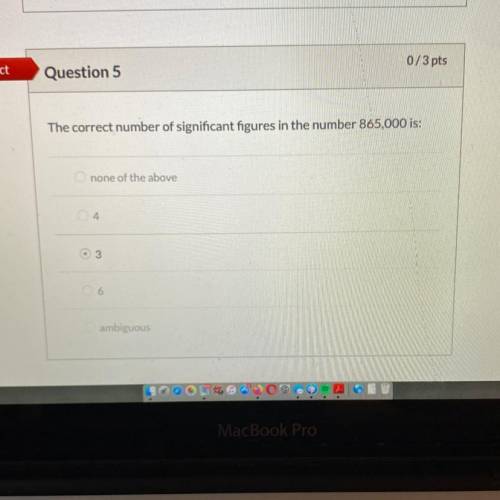

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1


Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1
You know the right answer?
Questions

Health, 10.12.2019 14:31


Mathematics, 10.12.2019 14:31

History, 10.12.2019 14:31



Geography, 10.12.2019 14:31

Physics, 10.12.2019 14:31




Physics, 10.12.2019 14:31


Advanced Placement (AP), 10.12.2019 14:31


History, 10.12.2019 14:31

Spanish, 10.12.2019 14:31

Mathematics, 10.12.2019 14:31

English, 10.12.2019 14:31



