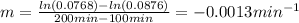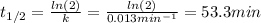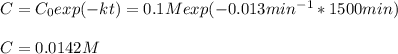3.The following data of decomposition reaction of thionyl chloride (SO2Cl2) were collected at a certain temperature and the concentration of SO2Cl2 were monitored as shown in the table.
SO2Cl2 (g) SO2 (g) + Cl2 (g)
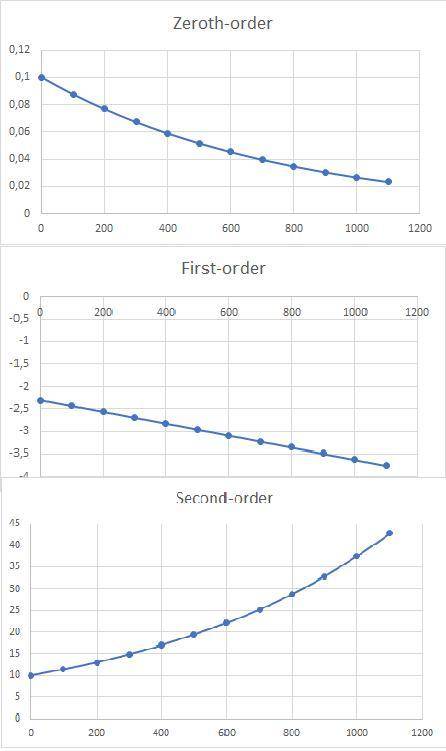
Time (min)Conc. of SO2Cl2 (mol/L)
00.1000
1000.0876
2000.0768
3000.0673
4000.0590
5000.0517
6000.0453
7000.0397
8000.0348
9000.0305
10000.0267
11000.0234
a)Determine graphically whether the kinetics of the reaction is zero order, first order or second order with respect to SO2Cl2 and then write the rate equation.
b)Determine the rate constant (k) of the reaction.
c)Determine the half-life (t½) for the reaction.
d)What will be the concentration of SO2Cl2 left in the reaction mixture at 1500 minutes?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
3.The following data of decomposition reaction of thionyl chloride (SO2Cl2) were collected at a cert...
Questions



History, 10.07.2019 13:30

Physics, 10.07.2019 13:30

Mathematics, 10.07.2019 13:30

Mathematics, 10.07.2019 13:30


Biology, 10.07.2019 13:30

Mathematics, 10.07.2019 13:30

Mathematics, 10.07.2019 13:30

Mathematics, 10.07.2019 13:30






Physics, 10.07.2019 13:40



History, 10.07.2019 13:40