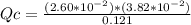Chemistry, 19.06.2020 03:57 ericchen4399
Consider the following reaction where Kc = 1.20×10-2 at 500 K. PCl5(g) PCl3(g) + Cl2(g) A reaction mixture was found to contain 0.121 moles of PCl5(g), 2.60×10-2 moles of PCl3(g), and 3.82×10-2 moles of Cl2(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals . The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Consider the following reaction where Kc = 1.20×10-2 at 500 K. PCl5(g) PCl3(g) + Cl2(g) A reaction m...
Questions


English, 03.06.2021 05:10


Mathematics, 03.06.2021 05:10


Mathematics, 03.06.2021 05:10



Computers and Technology, 03.06.2021 05:10


Mathematics, 03.06.2021 05:10

Mathematics, 03.06.2021 05:10

Mathematics, 03.06.2021 05:10

Business, 03.06.2021 05:10

Arts, 03.06.2021 05:10

Mathematics, 03.06.2021 05:10

Mathematics, 03.06.2021 05:10



![Qc=\frac{[C]^{c} *[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0689/6440/ada98.png)
![Qc=\frac{[PCl_{3}]*[Cl_{2} ]}{[PCl_{5} ] }](/tpl/images/0689/6440/81d8b.png)
![[PCl_{3} ]=\frac{2.60*10^{-2} moles}{1 liter} =2.60*10^{-2} \frac{moles}{L}](/tpl/images/0689/6440/fd446.png)
![[Cl_{2} ]=\frac{3.82*10^{-2} moles}{1 liter} =3.82*10^{-2} \frac{moles}{L}](/tpl/images/0689/6440/fced6.png)
![[PCl_{5} ]=\frac{0.121 moles}{1 liter} =0.121 \frac{moles}{L}](/tpl/images/0689/6440/529d8.png)