
Consider the following reaction where Kc = 0.159 at 723 K. N2(g) + 3H2(g) 2NH3(g) A reaction mixture was found to contain 1.97×10-2 moles of N2(g), 3.82×10-2 moles of H2(g) and 5.27×10-4 moles of NH3(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals 9.63x10^4 . The reaction b A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Consider the following reaction where Kc = 0.159 at 723 K. N2(g) + 3H2(g) 2NH3(g) A reaction mixture...
Questions







Mathematics, 11.02.2020 20:24








English, 11.02.2020 20:24


Chemistry, 11.02.2020 20:24



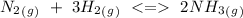
![K_e_q=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0689/0595/b557b.png)
![Q_c=\frac{[5.27X10^-^4]^2}{[1.97X10^-^2][3.82X10^-^2]^3}=0.251](/tpl/images/0689/0595/172a3.png)


