
Chemistry, 18.06.2020 07:57 joslynndiggs
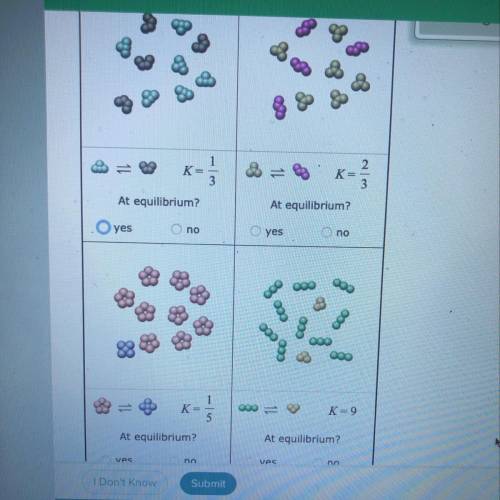
Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that individual molecules could be seen. (The water molecules are
not shown.)
The two substances in each sample can interconvert. That is, each kind of molecule can turn into the other. The equilibrium constant K for each interconversion
equilibrium is shown below the sketch.
Decide whether each solution is at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The wilson chamber is used to study: direction, speed, and distance of radioactivity the intensity of radiation the appearance of individual atoms all of the above
Answers: 1

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3
You know the right answer?
Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that indi...
Questions



History, 28.08.2020 23:01


History, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01

Spanish, 28.08.2020 23:01

Social Studies, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01






Health, 28.08.2020 23:01

Biology, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01

History, 28.08.2020 23:01

Mathematics, 28.08.2020 23:01



