
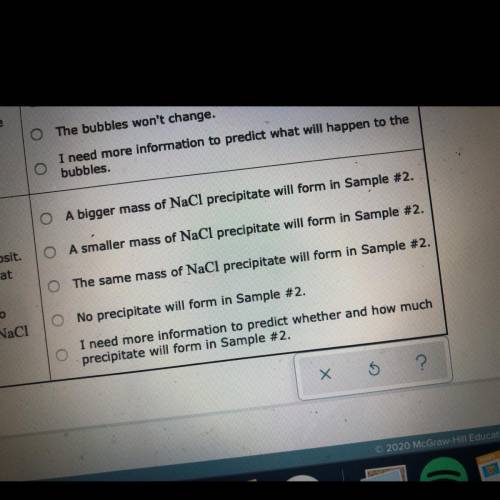
Two 250 mL samples of water are drawn from a deep
well bored into a large underground salt (NaCl) deposit. Sample #1 is from the top of the well, and is initially at 42 °C. Sample #2 is from a depth of 150 m, and is
initially at 8 °C. Both samples are allowed to come to room temperature (20 °C) and 1 atm pressure. An NaCl precipitate is seen to form in Sample #1.
The rest of the question is in the picture!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2


Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
Two 250 mL samples of water are drawn from a deep
well bored into a large underground salt (NaCl) d...
Questions



English, 13.11.2019 23:31


Mathematics, 13.11.2019 23:31


Social Studies, 13.11.2019 23:31







Mathematics, 13.11.2019 23:31

Social Studies, 13.11.2019 23:31








