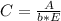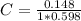Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
On a distance vs time graph the line of an object at rest is a
Answers: 1

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3
You know the right answer?
After creating a Beer's Law plot using standard solutions of Q, you determined the slope of Beer's L...
Questions


Chemistry, 22.03.2021 19:10


Mathematics, 22.03.2021 19:10

English, 22.03.2021 19:10

History, 22.03.2021 19:10

English, 22.03.2021 19:10

English, 22.03.2021 19:10

English, 22.03.2021 19:10


Chemistry, 22.03.2021 19:10





Social Studies, 22.03.2021 19:10

Biology, 22.03.2021 19:10



Computers and Technology, 22.03.2021 19:10


 . On this equation we will have:
. On this equation we will have: