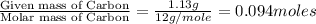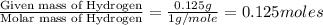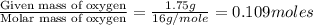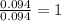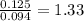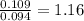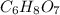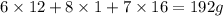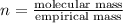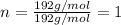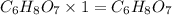A 3.00g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 192./gmol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 4.13g water 1.13g Use this information to find the molecular formula of X.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2


Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
A 3.00g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to hav...
Questions

Mathematics, 03.04.2020 03:14




Mathematics, 03.04.2020 03:15


Mathematics, 03.04.2020 03:15

Mathematics, 03.04.2020 03:15

History, 03.04.2020 03:15

English, 03.04.2020 03:15

English, 03.04.2020 03:15

English, 03.04.2020 03:15





Mathematics, 03.04.2020 03:15

Biology, 03.04.2020 03:15

Mathematics, 03.04.2020 03:15

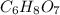
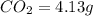
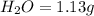
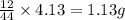 of carbon will be contained.
of carbon will be contained.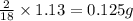 of hydrogen will be contained.
of hydrogen will be contained.