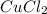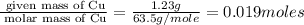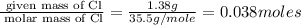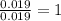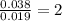Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

You know the right answer?
Copper metal of 1.23 g sample is reacted completely with chlorine gas to produce
2.61 g of copper c...
Questions


Mathematics, 13.05.2021 04:50


Mathematics, 13.05.2021 04:50

Mathematics, 13.05.2021 04:50

History, 13.05.2021 04:50



Mathematics, 13.05.2021 04:50


Arts, 13.05.2021 04:50


Mathematics, 13.05.2021 04:50


Social Studies, 13.05.2021 04:50



Mathematics, 13.05.2021 04:50