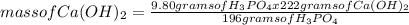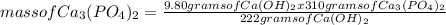Chemistry, 16.06.2020 07:57 perezsamantha3oqr0za
What is the maximum amount of Ca3(PO4)2 that can be prepared from 9.80 g of Ca(OH)2 and 9.80 g of
H3PO4
Ca(OH)2 (s) + H3PO4 (aq)
Ca3(PO4)2 (aq) + H2O (1)
balance the equation 1st.
O 6.80 g
O 15.5 g
O 8.60 g
o 13.7 g
O 10.3 g

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
What is the maximum amount of Ca3(PO4)2 that can be prepared from 9.80 g of Ca(OH)2 and 9.80 g of
H...
Questions

Arts, 14.07.2019 01:00



Arts, 14.07.2019 01:00


Arts, 14.07.2019 01:00



Chemistry, 14.07.2019 01:00

Arts, 14.07.2019 01:00

Arts, 14.07.2019 01:00



Mathematics, 14.07.2019 01:00


Mathematics, 14.07.2019 01:00

Mathematics, 14.07.2019 01:00

Mathematics, 14.07.2019 01:00

Mathematics, 14.07.2019 01:00