
Chemistry, 14.06.2020 14:57 kenisonpaigebosma
Chlorine dioxide is used as a disinfectant and bleaching agent. In water, it reacts to form chloric acid (HClO3),
according to the following balanced equation:
6 ClO2 + 3 H2O → 5 HClO3 + HCl
If excess ClO2 is mixed with 18.0 mL of H2O (d = 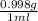 ) how many grams of HClO3 are formed?
) how many grams of HClO3 are formed?
PLEASE DO NOT JUST GIVE THE ANSWER!!! I really would like to learn HOW to do this so please give explanations for each step as you solve it. Thanks!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

You know the right answer?
Chlorine dioxide is used as a disinfectant and bleaching agent. In water, it reacts to form chloric...
Questions

Mathematics, 23.03.2021 19:30

English, 23.03.2021 19:30


Mathematics, 23.03.2021 19:30

Spanish, 23.03.2021 19:30

Physics, 23.03.2021 19:30

Geography, 23.03.2021 19:30

Mathematics, 23.03.2021 19:30


Chemistry, 23.03.2021 19:30

Social Studies, 23.03.2021 19:30




Mathematics, 23.03.2021 19:30

History, 23.03.2021 19:30

Spanish, 23.03.2021 19:30

Mathematics, 23.03.2021 19:30

Mathematics, 23.03.2021 19:30



